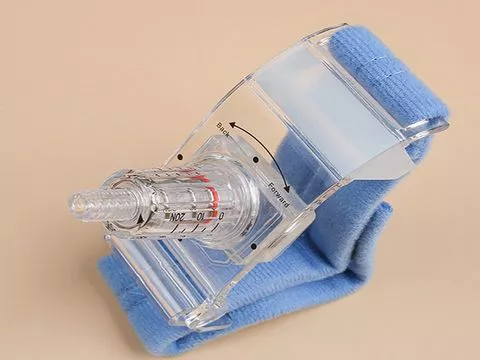
Description
Model : BR-RAT2
Material : Plastic + Cotton
Classification : Class I
Type:screw
Certification: ISO13485 / CE
Disinfect : EO gas
Disposable: Yes
Shelf life : 3 Years
Features:
The device is intended for use with hemostasis after the radial arterial catheterization
FOB port : Shenzhen other
Accept OEM Order!
- Radial ArteryTourniquets
- Cardiovascular Interventional Surgery
Production Capacity:
5000
Delivery Timeframe:
Within 60 Days
Incoterms:
CIF - Cost, Insurance and Freight
EXW - Ex Works
FOB - Free on Board
Packaging Details:
Not informed
More about
Brun Medical Co.,Ltd.
100-200
Employees
1M - 2M
Sales volume (USD)
10%
% Export sales
Year
Established
Business type
- Industry / Manufacturer
- Distributor / Wholesaler
Keywords
- medical OEM manufactuer
- interventional accessories
- balloon inflation device
- introducer sheath sets
- manifolds
Contact and location
-
Rosy ********
-
+86 1********
-
Zhuzhou / Hunan | China